Thursday, June 30, 2022

Author, Lead Researcher: Christopher L. Kepley, PhD, FAAAAI
Pictures: Chris Breedlove, LUCOM Marketing

Liberty University College of Osteopathic Medicine (LUCOM) faculty researchers have described and tested a new strategy for cancer immunotherapy that utilizes an alternative cell type for seeking out and destroying cancer cells. Using pre-clinical models, this method uses a patient’s own fat or blood cells to grow into other cells that are loaded with tumor killing molecules that are then targeted to the cancer cells using highly specific antibodies. Using this strategy, the tumor killing cells only release these tumor killing molecules when they encounter cancer cells, leaving other healthy cells alone. These studies led by Christopher L. Kepley, PhD, FAAAAI, professor of immunology, along with his colleagues from the National Institutes of Health and the University of North Carolina, were recently published in Frontiers in Oncology.
Adoptive cell transfer for cancer immunotherapy is one of the most exciting areas of cancer research. This strategy is typified by the use of autologous, peripheral T cells engineered ex vivo to express a transmembrane chimeric antigen receptor composed of an extracellular, antigen-specific single-chain antibody and an intracellular T cell signaling domain (CAR T). The use of CAR T-cell therapies has been approved by the Food and Drug Administration for children with acute lymphoblastic leukemia and adults with advanced lymphomas. The disadvantage is that CAR-T therapies have had less success with solid tumors and can also have unwanted toxicities.
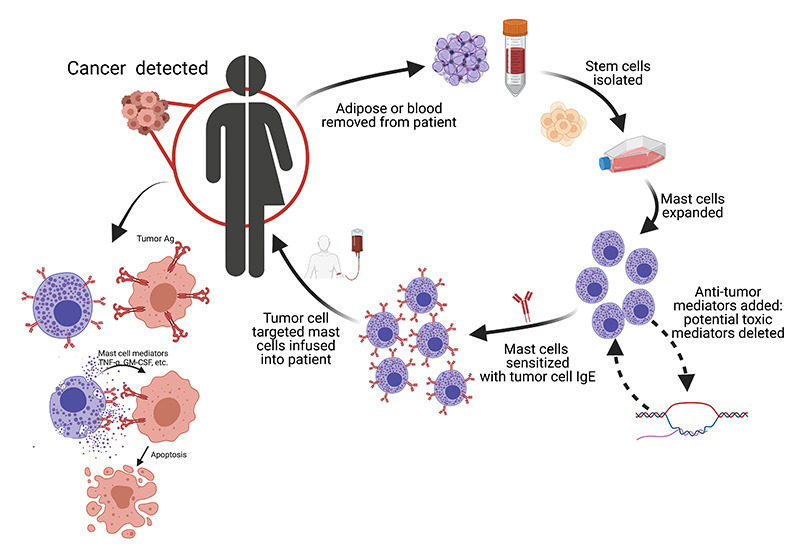
There is still an unmet need in cellular-based cancer immunotherapy for new strategies with diverse anti-tumor mechanisms for use alone or in combination with established protocols. Mast cells are found in most tissues and it has been controversial as to whether they play a pro-or anti-tumorigenic role. However, they are unique in that they have prestored, releasable tumor necrosis factor alpha (TNF-α) which, as the name implies, has anti-tumor activity. Mast cells also store and release several other potential anti-tumor mediators. Researchers and colleagues at LUCOM developed an approach to direct anti-tumor mediators in mast cells against cancer cells bearing specific molecules on their cell surface. Using this “Trojan Horse” strategy the mast cells bind only to the cancer cells and directly release their tumor killing molecules leaving healthy cells intact.
They did so by first developing methods so that the human mast cells could be differentiated from a patient’s own fat cells or from their blood. This was required because the immune system would recognize and destroy the mast cells if they were from another donor. The mast cells are expanded ex vivo so that there enough for re-injection into the patient. They used humanized antibodies that bind on one end to antigens found highly upregulated on cancer cells-in these studies HER2/neu a marker for certain breast cancers-that also bound on the other end to the mast cells with a very high affinity. The antibody also serves as a “trigger” to release all of the anti-tumor mediators at and within the cancer cells.

LUCOM faculty researchers demonstrated that the mast cells are safe for injection even at very high numbers. Mast cells with the HER2/neu antibody bound to and induced the death of tumor cells in both tissue culture and mouse models of human cancer. The control mast cells, without the HER2/neu antibody, had no effect. The researchers also showed that the human tumors implanted in immunocompromised mice that were exposed to the mast cells were significantly smaller and the mice lived significantly longer. Importantly, the researchers also defined the conditions needed so that potential toxic mediators could be taken out and/or more potent anti-tumor mediators added to the mast cells. A schematic of this autologous mast cell cancer immunotherapy (AMCIT) is shown.
The strategy has several advantages over current ones as mast cell activation is hypothesized to induce acute inflammation in the tumor microenvironment and provoke the immune system to attack the cancer cells. Also, the potential to circumvent challenges associated with current adoptive cellular strategies (e.g. CAR T) in which hyperactive T cells can spiral out of control creating a well-documented “cytokine storm” associated with systemic cytokine release. Another benefit of this type of immunotherapy would be that it there is evidence that the mast cells can invade the inside of tumors to release their anti-tumor mediators. LUCOM researchers are now examining other types of tumors and exploring the safety of this approach; critical requirements to enter AMCIT into Phase 1 clinical trials. These studies were supported by a grant from the National Cancer Institute.
For current LUCOM faculty research programs, please visit LUCOM Center for Research.