Johns Hopkins Fellows visit FDA, learn impact of new technology on public health regulation
February 17, 2026 : By Ryan Klinker - Office of Communications & Public Engagement
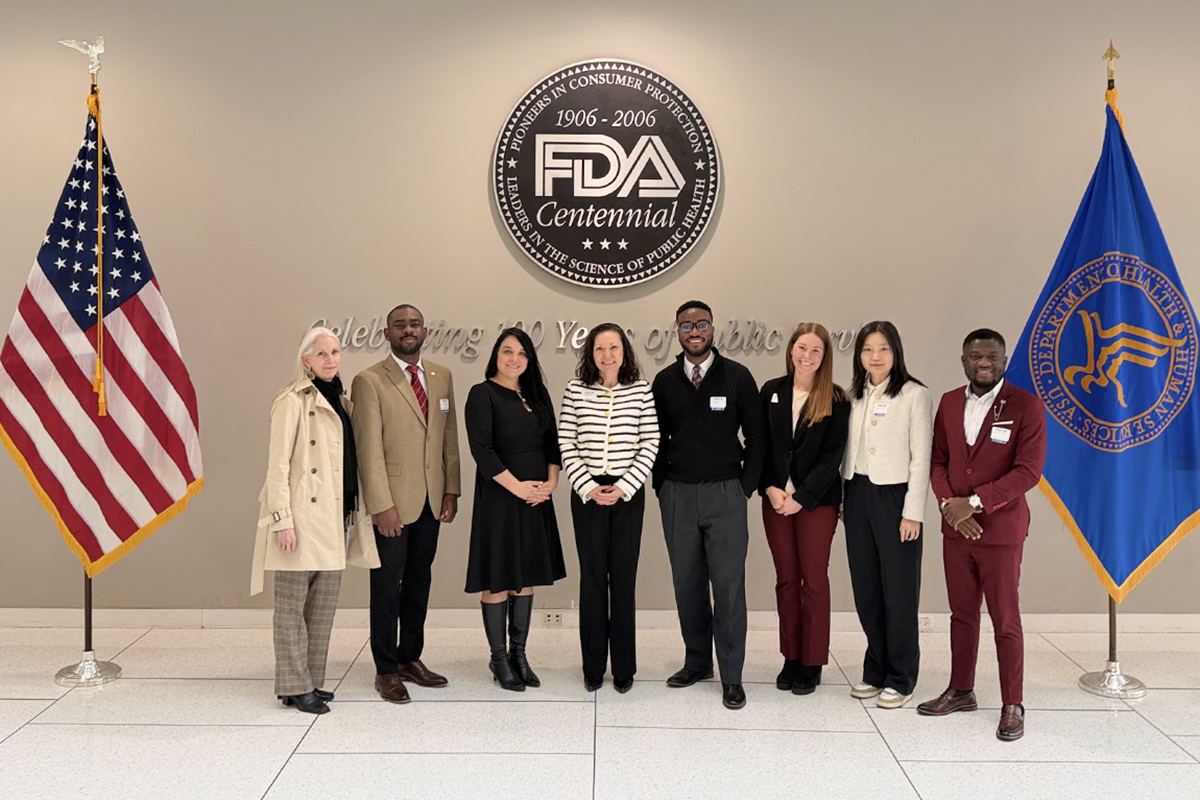
Students in the Johns Hopkins Fellowship program with Liberty University’s School of Health Sciences Department of Public Health recently visited the U.S. Food and Drug Administration in Maryland to gain rare insight into how the agency is modernizing regulatory science in response to rapid technological innovation that has the potential to shape the future of public health regulation, including artificial intelligence, real-world data, and technical transformation.
The fellowship is a collaborative partnership between Liberty University School of Health Sciences and the Johns Hopkins School of Medicine that provides selected graduate-level public health students with immersive experiences designed to strengthen research skills, foster interdisciplinary collaboration, and support professional research dissemination through publications and conference presentations. The partnership is in its third year.
During the trip to the FDA, current research fellows Bright Agbotui, Grace Sibert, and Raymond Suonyir were joined by two former fellows Miebaka Roberts (’25) and Andrea Mackenzie (’23) a current Ph.D. Public Policy student. Faculty and administrators from both schools also participated — Dr. Chen Dun, a faculty member and research associate at Johns Hopkins; Dr. Heidi DiFrancesa, dean of Liberty’s School of Health Sciences; and Christi Walsh, a former director of clinical research and nurse practitioner at Johns Hopkins.
“Experiences like this FDA visit equip our fellows with a firsthand understanding of how scientific innovation directly shapes regulatory decision‑making,” DiFrancesca said. “As emerging public health professionals, they are developing the analytical and leadership competencies required to operate at the highest levels of public health. Visits such as this reinforce their ability to translate complex data into thoughtful, responsible policy solutions.”
The fellows had the honor of meeting with FDA Commissioner Dr. Marty Makary, Chief Artificial Intelligence Officer Jeremy Walsh, FDA Senior Advisor and Medical Device Policy Officer Jared Seehafer, and Deputy Chief Medical Officer Dr. Mallika Mundker.
“As an aspiring public health leader, the experience at the FDA reinforced my understanding that effective leadership is grounded in the ability to translate rigorous evidence into sound policy,” Suonyir said. “My professional and leadership trajectory was greatly influenced by the chance to interact directly with public health experts at the highest levels, including FDA leadership. Seeing the deliberate, proactive measures being taken to protect public health inspired me, and this experience has made me even more determined to promote laws that enhance the health of the American people (wholistically).”
Fellows gained knowledge of the FDA’s initiatives to improve public communication, establish real-time reporting of adverse events, and centralize operations to increase efficiency. Additionally, the agency disclosed how FDA staff are collaborating with artificial intelligence to expedite pre-market review schedules while enhancing real-time post-market surveillance and safety analysis.
“The FDA’s prioritization of radical transparency showed how essential trust and credibility are in public health,” Sibert said. “The convergence of policy and practice in bolstering health systems was highlighted by the (transparency efforts) through programs such as real-time reporting.”
By engaging directly with national leaders, the student fellows strengthened the analytical and leadership skills essential for shaping evidence‑driven policy.
“In 2025, the FDA’s modernization of regulatory science reflects a decisive shift from siloed oversight to an integrated, data-driven regulatory ecosystem,” Agbotui said. “By restricting performative ‘singing and dancing’ styles in drug advertising, increasing real-time adverse-event tracking, and utilizing artificial intelligence to assist administrative review and clinical trial monitoring, the FDA has improved oversight. The thoroughness, openness, and scope of the work being done at the FDA to safeguard public health in an increasingly complicated biological environment greatly struck me.”
The visit further demonstrated how the Johns Hopkins Fellowship is helping Liberty’s public health students understand intricate healthcare and regulatory systems, participate in evidence-based research, and seek leadership positions in public health and health policy.
“Our students are learning to think critically at the intersection of science, technology, and regulation,” DiFrancesca said. “Their engagement at the FDA reflects both their growing competence and their commitment to advancing public health with integrity and purpose.”